STMicroelectronics Works with Alifax on Rapid, Cost-Efficient Point-of-Care Medical Testing
- Alifax, a diagnostic-test producer, has customized biological content and reagents to detect pandemic-related genetic material and other viral (and bacterial) pathogens
- ST Real-Time PCR molecular-diagnostics technology, using disposable chip-based cartridges, can be adapted to detect any genetic material
Stay tuned
To receive frequent updates via email, subscribe to our press releases.
 STMicroelectronics (NYSE: STM), a global semiconductor leader serving customers across the spectrum of electronics applications, and Alifax S.r.l, a producer of clinical diagnostic instrumentation, have worked together on a rapid, cost-efficient portable solution that will be available from Alifax, for point-of-care molecular diagnostic detection using highly reliable real-time Polymerase Chain Reactions (PCR) to amplify genetic material (RNA and DNA1) in patient samples.
STMicroelectronics (NYSE: STM), a global semiconductor leader serving customers across the spectrum of electronics applications, and Alifax S.r.l, a producer of clinical diagnostic instrumentation, have worked together on a rapid, cost-efficient portable solution that will be available from Alifax, for point-of-care molecular diagnostic detection using highly reliable real-time Polymerase Chain Reactions (PCR) to amplify genetic material (RNA and DNA1) in patient samples.
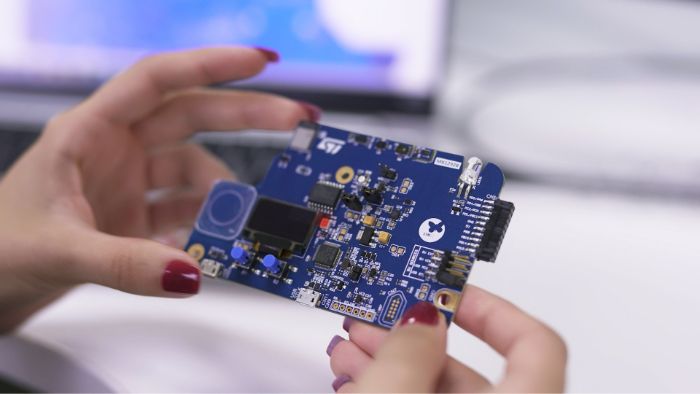
Leveraging technology developed and licensed from ST, Alifax is manufacturing the Molecular Mouse, a small portable instrument that contains a broad range of ST components, including STM32 MCUs, sensors, amplifiers, and other devices. Connected to a PC, the Molecular Mouse uses medical reagents2 created by Alifax to manage the control and testing of multiple targets or samples on a tiny disposable cartridge, manufactured by ST using its high-volume Micro-Electro-Mechanical Systems (MEMS) semiconductor process technology. Test results are available in less than an hour.
“One crucial lesson from the current global pandemic is the importance of rapid, cost-efficient point-of-care testing that allows immediate remote diagnosis, and then, if necessary, patient isolation,” said Alessandro Cremonesi, Chief Innovation Officer, STMicroelectronics. “ST has been investing in its Real-Time PCR platform convinced that innovative semiconductor-based diagnostic solutions can positively impact our lives.“
“Leveraging our high-volume semiconductor-manufacturing technology and long-term leadership in microfluidics, we’ve developed a rapidly customizable, highly flexible cartridge and instrumentation platform that delivers rapid and precise point-of-care diagnostic results, which Alifax has used to respond almost immediately to present pandemic and future diagnostic needs,” added Benedetto Vigna, President Analog MEMS, and Sensors Group, STMicroelectronics.
ST has demonstrated its efficacy and efficiency to scale reliable, high-volume manufacturing of semiconductors and its expertise in microfluidics for more than 25 years. Moreover, roughly 10 years of proven success in developing MEMS-based Real-Time PCR technologies has now culminated in a product generation that Alifax has rapidly adapted to address the current global pandemic.
“Building on our passion for research and excellence in innovation, Alifax has established its strength in hematology and bacteriology for diagnostic purposes. By working closely with ST and combining its foundational microfluidic and other technology in the Molecular Mouse and our pathogen-specific assays, starting from a COVID-19 test, we’re ready to contribute to rapidly diagnosing, isolating, and ultimately stopping the spread of pathogens,” said Paolo Galiano, President Alifax.
Certified to CE-IVD3 the Molecular Mouse, disposable test cartridges, and assays are available now from Alifax.
You can watch a video on Molecular Mouse at https://www.alifax.com/products/nuova-category-page/show/molecular-mouse.
Further Technical Information
With a footprint4 smaller than a smartphone, the Alifax Molecular Mouse can run real-time quantitative Polymerase Chain Reactions (qPCR) on small disposable cartridges, manufactured by ST using its high-volume semiconductor manufacturing process technology.
Each cartridge features 6 tiny reaction chambers (for the COVID-19 test, Alifax is using 2 chambers per sample). Each chamber supports precise heating and cooling of the reagents and samples to amplify the target genetic material, if it is present.
At the same time, exploiting the customized reagents that feature fluorescent tags developed by Alifax specifically to detect COVID-19, the Molecular Mouse determines the presence of the target genetic material in each chamber based on fluorescence intensity on multiple wavelengths.
About STMicroelectronics
At ST, we are 46,000 creators and makers of semiconductor technologies mastering the semiconductor supply chain with state-of-the-art manufacturing facilities. An independent device manufacturer, we work with our 100,000 customers and thousands of partners to design and build products, solutions, and ecosystems that address their challenges and opportunities, and the need to support a more sustainable world. Our technologies enable smarter mobility, more efficient power and energy management, and the wide-scale deployment of the Internet of Things and 5G technology. Further information can be found at www.st.com.
 About Alifax
About Alifax
Alifax was founded in 1988 in Padova by Paolo Galiano as a result of his valuable experience in the laboratory diagnostic market with special reference to hematology, microbiology, serology and autoimmunity fields. For more information visit www.alifax.com
For Press Information Contact:
Michael Markowitz
Director Technical Media Relations
STMicroelectronics
Tel: +1 7 81 591 0354
Email: michael.markowitz@st.com
Alifax S.r.l.
Email: molecularmouse@alifax.com
1Ribonucleic Acid (RNA) and Deoxyribonucleic Acid (DNA) are the two main types of genetic material on which all life depends. DNA is the blueprint of life that provides the code for every cell’s activities. RNA converts that code into the instructions for making the proteins that support the structure, function, and regulation of the body’s tissues and organs. Identifying and recognizing specific RNA and DNA “codes” can detect specific pathogens.
2Alifax had been developing 4-5 assay panels for Sepsis, and one assay to detect Zika, Dengue, Chikungunya when the COVID-19 pandemic began. Reallocating resources, they’ve rapidly developed COVID-19 assays. The Sepsis and Zika, Dengue, and Chikungunya assays will follow soon.
3In-Vitro Diagnostic Medical Device Directive (98/79/EC)
4Weighing just 300g (~0.66 lbs.), the Molecular Mouse has a 14cm x 7cm footprint and is 8.5cm tall.
5The 46.9mm x 21.9mm x 8.2mm cartridge is smaller than a microscope slide.
Stay tuned
To receive frequent updates via email, subscribe to our press releases.